|
|
Hybrigenics, Paris

Hybrigenics is a biotechnology and pharmaceutical company focusing its internal R&D programs on innovative target and therapeutics against cancer and commercializing its high throughput technology and bioinformatics platforms to identify, validate and inhibit protein interactions. Hybrigenics is organized in two operational units: Hybrigenics Pharma for the internal R&D programs and Hybrigenics Services for the commercial business, coordinated with economies of scale by a common corporate, financial and administrative management. The core link among all Hybrigenics activities is trust in science for life.
Hybrigenics Pharma most advanced development program is based on inecalcitol, a vitamin D analogue, for prostate cancer in combination with current reference treatments, for improved efficacy and better tolerance. Hybrigenics Services offers to researchers from all life sciences an access to its ISO 9001-certified Yeast-Two Hybrid (Y2H) high throughput screening platform [1], to its sophisticated bioinformatics set of tools and extensive database, and to its chemical library and high throughput small molecule screening platform, as fee-for-service activities. Knowledge of protein-protein interaction networks is essential for the understanding of cell biology regulation and signaling [2,3]. Novel protein interactions can thus be identified in all life science areas and validated both in vitro and in cells using HTRF® systems. The Small Molecule Screening (SMS) service is an integrated solution from assay development to compound evaluation. Protein-protein interactions assays can be customized and screened with our highly diverse and natural compound libraries. We can also transfer customer-developed assays in our process, not only to screen protein interactions but also proteases and many more enzymes and receptors. Hybrigenics Pharma research program explores the role of Ubiquitin-Specific Proteases (USPs) in the degradation of onco-proteins, and the use of proprietary USP inhibitors against various cancer types.
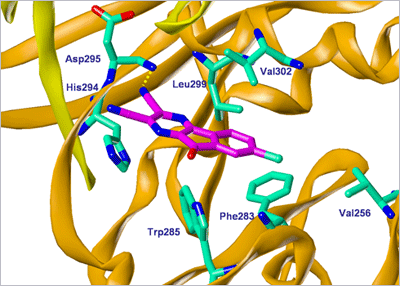
Screenshot of HBX 41,108 docked in USP7 structure as a possible Protein-Ligand model.
It is one of the first companies to have discovered potent and selective small molecule inhibitors of USP. USPs are involved in the deubiquitination of specific target substrates regulating their stability, subcellular localization and/or activation status. These proteins represent a druggable target class due to their thiol-protease catalytic core which is amenable to pharmacological inhibition by small molecules. Using USP protein networks and functional RNAi screening of genome wide USP, USP7/HAUSP and USP8 have been identified as key targets. More specifically, USP7 was reported to be involved in oncogene stabilization and inactivation of several tumour-suppressor genes resulting in cancer-cell proliferation and tumour agressiveness which makes USP7 a very promising therapeutic target. Advanced High-Throughput-Screening-compatible assays using optimized USP substrates were developed at Hybrigenics to screen our chemically diverse library from which several series of active molecules were found. We have previously shown the anti-cancer potential of HBX 41,108 [4] and of HBX 90,397 as an USP7 and USP8 inhibitor, respectively. Our drug discovery group also recently identified new promising chemical series exhibiting USP7-specific inhibition. Current attempts to develop specific and effective drugs targeting deubiquitinating enzymes will facilitate subsequent investigation of the role of this class of molecular targets in normal and disease states, and will provide a structural basis for drug development. We have therefore demonstrated the capacity of Hybrigenics to build a unique expertise in the molecular and cell biology, enzymology and pharmacology of USPs as well as a solid patent portfolio covering advanced screening assays and original small-molecule inhibitors.
Information and contact:
Dr Rémi Delansorne CEO
3-5 Impasse Reille
75014 PARIS, France
Web site: www.hybrigenics.com
Selected publications:
- [1] "The protein-protein interaction map of Helicobacter pylori", Rain JC, et al., Nature. (2001) 409(6817):211-5.
- [2] "Functional proteomics mapping of a human signaling pathway" Colland F, et al., Genome Res. (2004) 14(7) :1324-32.
- [3] "Protein interaction mapping: a Drosophila case study.", Formstecher E, et al., Genome Res. (2005) 15(3):376-84.
- [4] "Small-molecule inhibitor of USP7/HAUSP ubiquitin protease stabilizes and activates p53 in cells", Colland F, et al., Mol Cancer Ther. (2009) 8(8):2286-95.
|
|

Editor
Gabriele Costantino
Univ. Parma, IT
Editorial Committee
Erden Banoglu
Gazi Univ., TR
Leonardo Scapozza
Univ. of Geneve, CH
Wolfgang Sippl
Univ. Halle-Wittenberg, DE
Kristian Stromgaard
Univ. Copenhagen, DK
Mark Lansdell
Pfizer, UK

Executive Committee
Gerhard Ecker President
Roberto Pellicciari Past-Pres.
Koen Augustyns Secretary
Rasmus P.Clausen Treasurer
Javier Fernandez Member
Mark Bunnage Member
Peter Matyus Member

|









