|
|

Gerd Wagner |
Young researcher
Gerd Wagner is currently a Senior Lecturer in Medicinal Chemistry at King’s College London. Originally from beautiful South Germany, Gerd holds a degree in Pharmacy from the University of Freiburg and a PhD in Medicinal Chemistry (advisor: Professor Stefan Laufer) from the University of Tuebingen – two of Germany’s oldest and most prestigious academic institutions. In 2002, he joined the group of Professor Barry Potter at the University of Bath (UK) for postdoctoral studies on the role of cADPR and related dinucleotides in calcium signalling. He stayed in the UK to start his independent academic career, taking up a lectureship in Medicinal Chemistry at the University of East Anglia in 2004. In 2010, he moved to his current position at King’s College, where he is based in the Institute of Pharmaceutical Science. He is also the Head of the Institute’s Chemical Biology Unit and is chairing the steering group for the development of a new undergraduate programme “Chemistry with Biomedicine”, which is scheduled to launch at King’s in 2012.
Gerd’s main research interests are in medicinal chemistry and chemical biology. Research in the Wagner laboratory is concerned with the design, development and application of chemical tools to address important biological and biomedical questions, particularly in the area of glycobiology. The Wagner group currently occupies a large laboratory overlooking London’s South Bank, which is well equipped for synthetic and bioanalytical chemistry as well as protein biochemistry. Gerd’s research has been supported by the EPSRC, the MRC, the BBSRC, the Royal Society, the Leverhulme Trust and the Nuffield Foundation, and he collaborates successfully with research groups in the UK, Denmark and Germany.

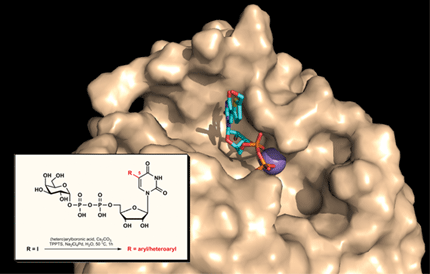
Glycosyltransferases as drug targets. Glycosyltransferases (GTs) are a large family of carbohydrate-active enzymes which transfer a sugar from a glycosyl donor to a suitable acceptor. GTs play a key role in many biological processes underpinning human health and disease, including glycoprotein and cell wall biosynthesis in human pathogens, carcinogenesis, and cellular adhesion. The considerable potential of GTs for drug discovery is undisputed, especially in therapeutic areas such as infection, inflammation and cancer. However, realising this potential has been hampered by a lack of potent, drug-like GT inhibitors. The Wagner group has recently discovered, in collaboration with Monica Palcic (Copenhagen) a novel type of GT inhibitor which targets the conformational plasticity of these enzymes (Nat Chem Biol 2010, 6, 321-323). The structural and enzymological information gleaned from these studies is currently being used for the rational development of 2nd generation inhibitor chemotypes with suitable properties for cellular studies and, potentially, drug development.
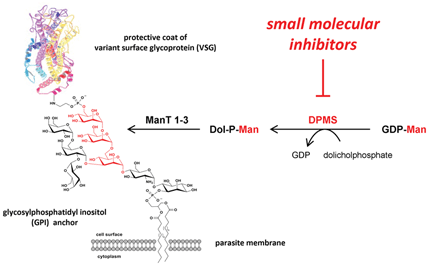
African Sleeping Sickness. African Sleeping Sickness is a devastating parasitic disease caused by the protozoon Trypanosoma brucei which threatens millions of people in sub-Saharan Africa. Current treatment options are limited, outdated and increasingly ineffective. The Wagner group is pursuing a variety of approaches to identify new therapeutic strategies for combating African Sleeping Sickness. The group has a longstanding interest in the development of drug-like inhibitors for parasitic glycosyltransferases involved in the biosynthesis of glycosylphosphatidyl inositol (GPI) anchors, which are essential for parasite viability. In collaboration with Terry Smith (St Andrews), Gerd and his co-workers have recently identified the first small molecular inhibitors of GPI anchor biosynthesis (Bioorg Med Chem Lett 2009, 19, 1749-1752). More recently, the group has also started to explore the potential of iron chelators as novel anti-parasitic agents (with Bob Hider, King’s College).
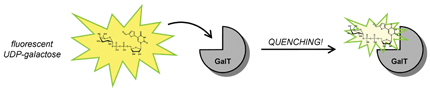
Synthetically modified biomolecules. The Wagner group has a proven track record in developing synthetic methodology for the direct structural modification, by cross-coupling chemistry, of sensitive biomolecules such as nucleotides, sugar-nucleotides and amino acids. Obviating the need for protecting groups and lengthy synthetic sequences, this synthetic approach has provided rapid access to structural analogues of naturally occurring biomolecules with interesting biological and biophysical properties. Cross-coupled derivatives of UDP-galactose, for example, are useful as broadly applicable fluorescent probes for glycosyltransferase ligand-displacement assays (ChemBioChem 2010, 11, 1392-1398). A similar synthetic strategy has led to the discovery of base-modified NAD derivatives which act as isoform-selective inhibitors of human sirtuins, NAD-dependent histone deacetylases that are emerging as promising anti-cancer targets (J. Med. Chem. 2011, in print).
Key references
- T. Pesnot, J. Kempter, J. Schemies, G. Pergolizzi, T. Rumpf, U. Uciechowska, W. Sippl, M. Jung, G. K. Wagner Two-step synthesis of novel, bioactive derivatives of the ubiquitous cofactor nicotinamide adenine dinucleotide (NAD). J. Med. Chem. 2011, in print.
- T. Pesnot, R. Jørgensen, M. M. Palcic, G. K. Wagner Structural and mechanistic basis for a new mode of glycosyltransferase inhibition. Nat. Chem. Biol. 2010, 6, 321-323.
- T. Pesnot, M. M. Palcic, G. K. Wagner A novel fluorescent probe for retaining galactosyltransferases. ChemBioChem 2010, 11, 1392-1398.
- G. K. Wagner, T. Pesnot Glycosyltransferases and their assays. ChemBioChem 2010, 11, 1939-1949. (review article)
- T. K. Smith, B. L. Young, H. Denton, D. L. Hughes, G. K. Wagner First small molecular inhibitors of T. brucei dolichol phosphate mannose synthase (DPMS), a validated drug target in African sleeping sickness. Bioorg. Med. Chem. Lett. 2009, 19, 1749-1752.
- A. Collier, G. K. Wagner A fast synthetic route to GDP-sugars modified at the nucleobase. Chem. Commun. 2008, 20, 178-180..
Information and contact
Dr Gerd Wagner
King’s College London
Institute of Pharmaceutical Science
Franklin-Wilkins Building
150 Stamford Street
Tel: +44 (0)20 7848 4747
E-mail: gerd.wagner@kcl.ac.uk
Personal website: link
Links in text
|
|

Editor
Gabriele Costantino
Univ. of Parma, IT
Editorial Committee
Erden Banoglu
Gazi Univ., TR
Lucija Peterlin Masic
Univ. of Ljubljana, SI
Leonardo Scapozza
Univ. of Geneve, CH
Wolfgang Sippl
Univ. Halle-Wittenberg, DE
Sarah Skerratt
Pfizer, Sandwich, UK

Executive Committee
Gerhard F. Ecker President
Roberto Pellicciari Past Pres.
Koen Augustyns Secretary
Rasmus P. Clausen Treasurer
Javier Fernandez Member
Mark Bunnage Member
Peter Matuys Member

|









