Cyclofluidic: The Robot Drug Discovery Scientist

Drug Discovery
The process of discovering a drug is slow and expensive, with the average cost of developing a new molecular entity (NME) now estimated at $1.8 billion.
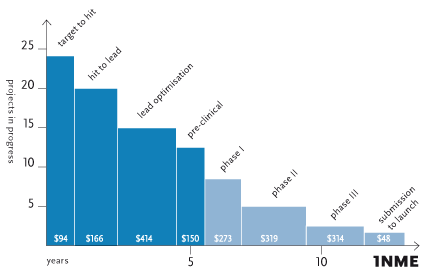
Drug Discovery pipeline to produce 1NME including cost per phase ($M)1
Recent analysis1 has shown that for every NME launched, 19.4 hit to lead programmes are required, at a cost per launch of $166 million, due to the high attrition rates in the drug discovery process. In addition, although much of this attrition (27%) is attributed to lack of efficacy in humans in phase II, the majority is reported to be related to safety, toxicology, formulation, pharmacokinetics and cost of goods (53%)2. These characteristics are defined by the chemical structure of the molecule itself.
The Importance of Getting the Right Lead Molecule
Studies3 have also shown that drug structures are very closely related to their leads and these leads to the hit series from which they were optimised, indicating that much of the success or failure of a potential drug molecule is laid down at this very early point in the drug discovery process. The decision on which lead series to pursue in the hit to lead phase is therefore crucial and often dictates the quality of the pre-clinical candidate and therefore the success of the programme and the time and resource required to launch.
Medicinal Chemistry
Small molecule lead discovery involves an iterative process of molecular design, chemical synthesis, biological assay and analysis to feed into the next learning cycle. In a typical hit to lead project, in vitro assays are used to measure the potency and selectivity of the molecule at the target of interest as well as a range of calculated and measured physical properties that help to predict a lead molecule's "drug-likeness". Using conventional approaches, each learning cycle in this process takes 1-8 weeks, depending on where compounds are made and tested. These cycle times lead to slow and expensive hit to lead exploration, limiting the number of lead series that can be assessed. In addition, compounds are often designed and made "at risk" i.e. without incorporating the latest data from previous design cycles, leading to wasted effort and resource. This means that lead optimisation can be long and costly and the choice of molecules available to be progressed to pre-clinical studies is sub-optimal.
The Cyclofluidic Approach
The Cyclofluidic technology platform integrates all these processes on the bench-top, allowing drug lead molecules to be assayed minutes (rather than weeks) after they are designed. Potential lead molecules are synthesised, purified and screened in fast serial mode, incorporating activity data from each compound as it is generated before selecting the next compound to make.
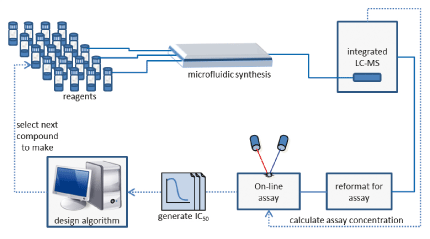
The Cyclofluidic Approach
To ensure data quality, each compound is purified by integrated high pressure liquid chromatography (HPLC), its identity confirmed by mass spectrometry and the concentration entering the assay determined in real time by evaporative light scattering detection (ELSD). The compound's IC50 is then measured in an on-line biochemical assay and this result fed into the design selection algorithm before the algorithm selects the next compound to make. The system is designed to use interchangeable design algorithms, assay formats and chemistries and at any stage a medicinal chemist can intervene in order to adjust the design strategy.
About Cyclofluidic
Cyclofluidic has been located in labs in Welwyn Garden City UK since early 2009 after being established as a joint pre-competitive investment between pharmaceutical companies UCB and Pfizer with a collaborative research grant from the UK Government Technology Strategy Board. The company was set up to leverage UK expertise in drug discovery and microfluidic technology in order to develop an integrated automated platform that significantly shortens the design-synthesis-test cycle from weeks to minutes allowing a step change in the ability to discover quality leads. The company is currently working with pharma and academic groups to apply its technology in tool and lead discovery projects.
_________________________________________________________________
Contact
Elizabeth Farrant
Email: elizabeth.farrant@cyclofluidic.co.uk
Phone: + 44 (0)1707 358706 /
+ 44 (0)7930 853662
Website: www.cyclofluidic.co.uk









