|
|

Oliver Werz |
LAB PRESENTATION
The Chair of Pharmaceutical/Medicinal Chemistry, Institute of Pharmacy, Univ. Jena, Germany
The current scientific interests of the Chair of Pharmaceutical/Medicinal Chemistry at the Friedrich-Schiller-University Jena comprise the identification and cellular regulation of potential drug targets, discovery of drugs, and characterisation of drugs actions with respect to inflammation and cancer. The various research activities can be grouped into three major areas: (I) investigation of pathobiochemical mechanisms of inflammation at the molecular and cellular level, (II) research and development of bioactive compounds that interfere with concrete drug targets, and (III) identification and validation of drug targets and signalling pathways of known bioactive compounds. The research projects are characterized by multiple and intensive scientific collaborations in a multidisciplinary manner ranging from computational chemistry, design, synthesis, biochemical and pharmacological analyses to clinical studies. The compounds of interest, provided and synthesized by collaborators, include diverse natural compound from plants, fungi and myxobacteria and semi-synthetic derivatives thereof but also series of compounds from synthetic origin. Particular focus is placed on sex differences in the biological regulation of drug targets and on the influence of sex and sex hormones on the pharmacology of bioactive compounds.
The group and head of the group
In 2010, the new Chair of Pharmaceutical/Medicinal Chemistry was installed when Oliver Werz moved together with one post-doc and threes PhD students from Univ. Tuebingen to Univ. Jena. Oliver Werz studied pharmacy and received his PhD degree at Tuebingen University in 1996. As a post-doc he gained particular knowledge on eicosanoids at Frankfurt University in Dieter Steinhilber´s lab and at the Karolinska Institute (Stockholm) in the lab of the Nobel Laureate Bengt Samuelsson. After the habilitation in 2002 and a subsequent senior scientist period at Frankfurt University, be became full professor for pharmaceutical analytics at the Pharmaceutical Institute, Tuebingen University in 2005.
Today, the group, managed by Oliver Werz, encompasses five senior scientists or post-docs (with partly independent research projects), nine PhD students, eight technicians and various diploma students. The group is intensively involved in local, national and international multidisciplinary research collaborations and consortia with academic and industrial partners. The labs are fully equipped with bioanalytical devices including live-cell fluorescence microscope, fluorescence scanner/reader and related imaging systems, automated HPLC systems, UPLC-MS/MS (triple quadrupole/ion trap) etc. One characteristic of the group is the availability of a broad variety of cell-free and cell-based assays for assessment of biological activities related to inflammation and cancer. The particular expertise on the identification of molecular targets for bioactive compounds by the target fishing approach and the discovery of dual 5-LO/mPGES-1 inhibitors as novel potential anti-inflammatory drugs are additional specific features. Finally, a cutting-edge role of the group has been proposed for considering sex differences as important variable in the early process of drug research and development, aiming for a “Gender-tailored therapy“.
Research area I
Pathobiochemical mechanisms of eicosanoids biosynthesis and sex differences
The biosynthesis of eicosanoids, in particular of leukotrienes via the 5-lipoxygenase (5-LO) pathway, is tightly regulated and a concerted action of many cellular factors, such as protein-protein interactions, phosphorylation, redox-tone, and cations or small messengers, determine the capacity to produce these mediators. Stably transfected HEK cells expressing mutated 5-LO and FLAP proteins are studied for protein (co-)localisation by fluorescence microscopy techniques and for 5-LO product release by UPLC-MS/MS and offer an excellent tool for analysis of modulation of the 5-LO pathway by endogenous and exogenous factors or pharmacological agents. The upstream signalling and the concrete molecular interactions are studied to understand the regulation of the synthesis of eicosanoid as well as to deduce novel concepts and aspects for improved pharmacological interference, in vitro and in vivo.
The incidence and severity of leukotriene-related disorders (e.g., asthma, allergic rhinitis) differs significantly between the genders but the biochemical mechanisms beyond this sex bias are unknown. Accordingly, we found that male sex due to higher testosterone suppresses leukotriene biosynthesis in vitro and in vivo by altering the subcellular localisation of 5-LO. We investigate the testosterone/ 5-LO signalling and we could show that extracellular signal-regulated kinases (ERK)-1/2 and phospholipase D are controlled in a sex-dependent manner. It is intriguing that the sex-dependent differential regulation of 5-LO causes differences in the molecular pharmacology and thus efficacy of some anti-leukotriene drugs.
Selected recent papers:
- Pergola, C., Rogge, A., Dodt, D., Northoff, H., Weinigel, C., Barz, D., Rådmark, O., Sautebin, L., Werz, O. (2011) Testosterone suppresses phospholipase D causing sex differences in leukotriene biosynthesis in human monocytes. FASEB Journal, 25, 3377-87
- Greiner, C., Hörnig, C., Rossi, A., Pergola, C., Zettl, H., Schubert-Zsilavecz, M., Steinhilber, D., Sautebin, L., and O Werz. (2011) 2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidin-2-ylthio)octanoic acid (HZ52) – a novel type 5-lipoxygenase inhibitor with favorable molecular pharmacology and efficacy in vivo Br. J. Pharmacol., 164, 781-93
- Feißt, C., Pergola, C., Koeberle, A., Dodt, G., Rakonjac, M., Hoffmann, M., Hoernig, C., Fischer, L., Steinhilber, D., Rådmark, O., Franke, L., Schneider, G., and Werz, O. (2009) Hyperforin inhibits 5-lipoxygenase by interference with the C2-like domain. Cell Moll Life Sci, 66, 2759-2771
- Pergola, C., Dodt, G., Rossi, A., Neunhoeffer, E., Lawrenz, B., Hinnak, H., Samuelsson, B., Rådmark, O., Sautebin, L., and Werz, O. (2008) ERK-Mediated Regulation of Leukotriene Biosynthesis by Androgens: A Molecular Basis for Gender Differences in Inflammation and Asthma. Proc Nat. Acad Sci USA, 105,19881-19886
- Albert, D., Pergola, C., Koeberle, A., Dodt, G., Steinhilber, D., and Werz, O. (2008) The role of diacylglyceride generation by phospholipase D and phosphatidic acid phosphatase in the activation of 5-lipoxygenase in polymorphonuclear leukocytes, J Leuk Biol, 83:1019-1027
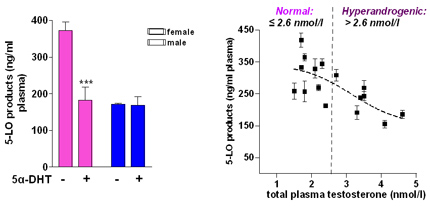
Testosterone suppresses 5-LO product synthesis and causes lower 5-LO product formation in males versus females. Left Panel: Formation of 5-LO products in stimulated blood from male (blue) and female (pink) donors and its suppression by 5α-dihydrotestosterone (5α-DHT) in female blood. Right Panel: 5-LO product formation is higher in females with low testosterone in plasma but is suppressed in hyperandrogenic females with high testosterone plasma levels.
Research area II
Inhibitors of microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase and related anti-inflammatory active agents
The research aims at identifying novel anti-inflammatory agents, as alternatives to non-steroidal anti-inflammatory drugs (NSAIDs) that block cyclooxygenases, able to inhibit the 5-lipoxygenase (5-LO) pathway or microsomal prostaglandin E2 synthase (mPGES)-1 but also compounds that dually block 5-LO and mPGES-1. The idea behind such pharmacological profiling is to obtain (i) selective and efficient enzymes inhibitors lacking the disadvantages of former drug candidates, (ii) effective anti-inflammatory agents with synergistic action against both prostaglandin E2 and leukotrienes, (iii) drugs with less side effects as compared to traditional NSAIDs. The discovery of potential compounds is guided by pharmacophore models and (natural) compound library screens as well as by identification of molecular mechanisms of known anti-inflammatory agents (synthetic or natural ones). The computer-aided design, the isolation and synthesis of compounds is performed together with specialized collaborators world-wide. Plenty of cell-free and cell-based assays are available but still are optimized for smart-screening. Investigations of the molecular pharmacological profile of inhibitors are conducted that consider in-vivo relevant influences of their action. Examples of successful developments are series of benzo[g]indole-3-carboxylates, triazoles, benzimidazoles, γ-hydroxybutenolides, and α-substituted pirinixic acid derivatives, and the natural compounds myrtucommulone, hyperforin, arzanol, boswellic acids and semisynthetic derivatives thereof, respectively.
Selected recent papers:
- Waltenberger, B., Wiechmann, K., Bauer, J., Markt, P., Noha, S.M., Wolber, G., Rollinger, J.M., Werz, O., Schuster, D., and Stuppner, H. (2011) Pharmacophore Modeling and Virtual Screening for Novel Acidic Inhibitors of Microsomal Prostaglandin E2 Synthase-1 (mPGES-1). J Med Chem, 54, 3163-74
- Banoglu, E., Çalışkan, B., Luderer, S., Eren, G., Özkan, Y., Altenhofen, W., Weinigel, C., Barz, D., Gerstmeier, J., Pergola, C., and Werz, O. (2012) Identification of novel benzimidazole derivatives as inhibitors of leukotriene biosynthesis by virtual screening targeting 5-lipoxygenase-activating protein (FLAP). Bioorg Med Chem, 20, 3728-41
- Pergola, C., Jazzar, B., Rossi, A., Northoff, H., Hamburger, M., Sautebin, L., and Werz, O. (2011) On the inhibition of 5-lipoxygenase product formation by tryptanthrin: mechanistic studies and efficiency in vivo. Br. J. Pharmacol, 165, 765-76
Hieke, M., Greiner, C., Dittrich, M., Reisen, F., Schneider, G.,
- Schubert-Zsilavecz, M., and Werz, O. (2011) Discovery and biological evaluation of a novel class of dual microsomal prostaglandin E2 synthase-1/5-lipoxygenase inhibitors based on 2-[(4,6-diphenethoxypyrimidin-2-yl)thio]hexanoic acid. J Med. Chem., 54, 4490-507
- Pergola, C., Jazzar, B., Rossi, A., Buehring, U., Luderer, S., Dehm, F., Northoff, H., Sautebin, L., Werz, O. (2011) Cinnamyl-3,4-dihydroxy-α-cyanocinnamate (CDC) is a potent inhibitor of 5-lipoxygenase. J Pharmacol Exp Ther, 338, 205-13
- Greiner, C., Hörnig, C., Rossi, A., Pergola, C., Zettl, H., Schubert-Zsilavecz, M., Steinhilber, D., Sautebin, L., and O Werz. (2011) 2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidin-2-ylthio)octanoic acid (HZ52) – a novel type 5-lipoxygenase inhibitor with favorable molecular pharmacology and efficacy in vivo Br. J. Pharmacol., 164, 781-93
- Koeberle, A., Rossi, A., Zettl, H., Pergola, C., Dehm, F., Bauer, J., Greiner, C., Reckel, S., Hoernig, C., Northoff, H., Bernhard, F., Dötsch, V., Schubert-Zsilavecz, M., Sautebin, L., and Werz, O.(2009) The molecular pharmacology and in vivo activity of YS121 {2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2-ylthio)octanoic acid}, a dual inhibitor of microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase, J Pharmacol Exp Ther, 332, 840-848
- Werz, O., Greiner, C., Koeberle, A., Hoernig, C., George, S., Popescu, L., Syha, I., Schubert-Zsilavecz, M., Steinhilber, D. (2008) Novel and potent inhibitors of 5-lipoxygenase product synthesis based on the structure of pirinixic acid. J Med Chem, 51, 5449-5453
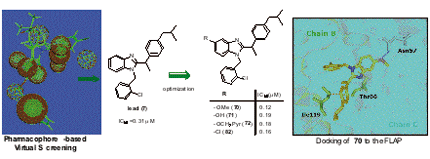
Virtual screening using a combined ligand- and structure-based pharmacophore model for 5-lipoxygenase-activating protein (FLAP) inhibitors led to the identification of 1-(2-chlorobenzyl)-2-(1-(4-isobutylphenyl)ethyl)-1H-benzimidazole (7) as developable candidate. Compound 7 potently suppresses leukotriene formation in intact neutrophils (IC50 = 0.31 µM) due to interaction with FLAP. A series of 46 benzimidazole-based derivatives of 7 were synthesized leading to more potent analogues (70-72, 82) with IC50 = 0.12 - 0.19 µM in intact neutrophils.
Research area III
Identification and validation of drug targets and signalling pathways of bioactive agents
Natural products of interest include plant-derived triterpenic acids (e.g., boswellic acids, tirucallic acids, lupanic acids), acylphloroglucinols (e.g., hyperforin, myrtucommulon), curcumin, EGCG and similar polyphenols (e.g., resveratrol) but also structurally more complex myxobacterial compounds (archazolid, pretubulysin and chondramide). We aim to identify the molecular targets and the related molecular/biochemical mechanisms as well as the pharmacological relevance of the drug/target interaction. The established methods for compound immobilisation and target-fishing are used for identification of (novel) targets of known bioactive agents (resveratrol, myrtucommulone, testosterone) or drugs (dexamethasone, atorvastatin) and the methodology is further optimized. Moreover, the suitability of these compounds as tools for investigating signalling pathways in cell-based models is of interest. In collaboration with others total syntheses of selected natural products (e.g., myrtucommulon, arzanol) are attempted in order to design semi-synthetic compound libraries for SAR studies and search for hits as well as to allow covalent linking to insoluble resins to be applied for target-fishing. The molecular/pharmacological evaluation strongly considers in vivo-relevant regulatory mechanisms in optimized test systems and is supposed to identify possible points of attack/target sites for the molecular interference (e.g., C2-domains). Such knowledge offers on one hand the development of advanced therapeutics and on the other hand enables to reveals novel protein functionalities in pathobiochemical processes (active agents as tools).
Selected recent papers:
- Verhoff, M., Seitz, S., Northoff, H., Jauch, J., Schaible, A.M., Werz, O. (2012) A novel C(28)-hydroxylated lupeolic acid suppresses the biosynthesis of eicosanoids through inhibition of cytosolic phospholipase A2. Biochemical Pharmacol., in press
- Henkel, A., Kather, N., Mönch, B., Northoff, H., Jauch, J., Werz, O. (2012) Boswellic acids from frankincense inhibit lipopolysaccharide functionality through direct molecular interference. Biochem Pharmacol., 83, 115-21.
- Golkowski, M., Pergola, C., Werz, O., and Ziegler, T. (2012) Strategy for catch and release of azide-tagged biomolecules utilizing a photolabile strained alkyne construct. Org Biomol Chem., 10, 4496-9
- Bauer, J., Koeberle, A., Dehm, F., Pollastro, F., Appendino, G., Northoff, H., Rossi, A., Sautebin, L., and Werz, A. (2011) Arzanol, a prenylated heterodimeric phloroglucinyl pyrone, inhibits eicosanoid biosynthesis and exhibits anti-inflammatory efficacy in vivo. Biochem Pharmacol, 81, 259-68
- Koeberle, S., Romir, J., Fischer, S., Koeberle, A., Schattel, V., Albrecht, W., Grütter, C., Werz, O., Rauh, D., Stehle, T., Laufer, S. (2011) Skepinone-L: a p38 mitogen activated protein kinase (MAPK) inhibitor with unsurpassed selectivity and outstanding in vivo efficiency. Nat. Chem. Biol., 8, 141-3
- Siemoneit, U., Koeberle, A., Rossi, A., Dehm, F., Reckel, S., Maier, T.J., Jauch, L., Northoff, H., Bernhard, F., Doetsch, V., Sautebin, L., and Werz, A. (2011) Boswellic acids inhibit microsomal prostaglandin E2 synthase-1: A molecular basis for the anti-inflammatory actions of the bioactive ingredients from frankincense Br. J. Pharmacol, 162, 147-62
- Tausch, L., Henkel, A., Siemoneit, U., Poeckel, D., Kather, N., Franke, L., Schneider, G., Angioni, C., Geisslinger, G., Skarke, C., Holtmeier, W., Beckhaus, T., Karas, M., Jauch, J., and Werz, O. (2009) Identification of human cathepsin G as a functional target of boswellic acids from the anti-inflammatory remedy frankincense. J Immunol, 183, 3433-3442.
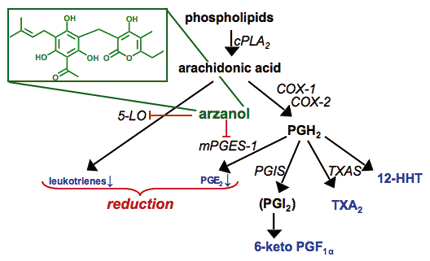
Arzanol, a prenylated acylphloroglucinol from Helichrysum italicum, as representative of several dual 5-LO/mPGES-1 inhibitors identified by Werz group. The compound blocks the synthesis of proinflammatory leukotrienes and PGE2 within the arachidonic acid cascade but leaves the formation of other important eicosanoids unaffected. cPLA2, cytosolic phospholipase A2; COX, cyclooxygenase; PG, prostaglandin; 5-LO, 5-lipoxygenase; mPGES-1, microsomal prostaglandin E2 synthase-1; TXA, thromboxane A; TXAS, thromboxane A synthase; PGIS, prostaccylin synthase.
_____________________________________________________
Contact Address:
Prof. Dr. Oliver Werz
Chair of Pharmaceutical/Medicinal Chemistry
Institute of Pharmacy,
University Jena
Philosophenweg 14
D-07743 Jena
Germany
Phone: +49-03641-949801
Fax: +49-03641-949802
E-mail: oliver.werz@uni-jena.de
http://www.uni-jena.de/pharm_med_chemie.html
|
|

Editor
Gabriele Costantino
Univ. of Parma, IT
Editorial Committee
Erden Banoglu
Gazi Univ., TR
Lucija Peterlin Masic
Univ. of Ljubljana, SLO
Leonardo Scapozza
Univ. of Geneve, CH
Wolfgang Sippl
Univ. Halle-Wittenberg, DE
Sarah Skerratt
Pfizer, Sandwich, UK

Executive Committee
Uli Stilz President
Gerhard F. Ecker Past Pres.
Koen Augustyns Secretary
Rasmus P. Clausen Treasurer
Hein Coolen Member
Gabriele Costantino Member
Phil Jones Member

|









