
Introduction to ELF
The goals of the IMI European Lead Factory (ELF) are to make available a high quality compound collection and to provide the extensive infrastructure to screen it against novel biological targets. These compounds, facilities and capabilities are available to a wide range of researchers including public partners (European academics and SMEs) and private partners.
For public partners, there is no cost associated with generating hits for their targets as funding is provided by the Innovative Medicines Initiative (IMI) although further exploitation of hits is subject to a number of conditions. The ELF also opens up the opportunity to contribute new ideas for compound libraries helping to make the compound collection highly innovative. Overall, the ELF offers an outstanding opportunity to the European research community to discover novel compounds for use as pharmacological tools or as the starting points for drug discovery projects.
Biological targets and ideas for compound libraries are being sought now and if you would like to take advantage of this opportunity or would like further information then visit the website: www.europeanleadfactory.eu
Background
The Innovative Medicines Initiative (IMI) is Europe's largest public-private partnership1, which aims to improve the drug development process by supporting more efficient discovery and development of better and safer medicines for patients. With a €2 billion euro budget, IMI supports collaborative research projects and builds networks of industrial and academic experts in Europe to encourage innovation in healthcare. IMI is a Joint Undertaking between the European Union and the European Federation of Pharmaceutical Industries and Associations (EFPIA).
IMI creates large-scale networks of innovation in pharmaceutical research. By joining forces in the IMI research and training projects, competing pharmaceutical companies collaborate not only with each other but also with academia, regulatory agencies and patients' organisations, in order to tackle major challenges in drug development.
The IMI Scientific Research Agenda sets out the research priorities of IMI, which are the basis for annual calls for proposals. The 5th call outlined the requirements for a European Lead Factory.
Goals of the European Lead Factory
The goals of the ELF are to assemble a Joint European Compound Collection (JECC) and to enable this collection to be screened for both public partners (Academics and SMEs) and contributing EFPIA partners. In addition, data will be collected and used to identify successful approaches to library design and high throughput screening.
JECC
The JECC is created from two sources. The first of these is contributions from pharma companies. More than 300,000 compounds have been selected from the collections of the seven EFPIA companies involved in the project. Recent publications2 outline the potential benefits of combining two company’s collections. Here, selections of compounds from seven companies have been combined to greatly expand the benefits of compound sharing. The chemical and biological profile of the collection will be described in forthcoming publications; in brief, compounds have been selected that are drug-like and synthetically tractable, and whose calculated properties suggest that they will make high quality starting points for further work. The selection process from the seven companies was iterative, and any duplicates were replaced by alternatives. A substantial compound collection derived from multiple sources has been assembled rapidly and is available for screening.
Bespoke Compounds
Compounds will also be synthesized specifically for the JECC - this is the second source of compounds. Ideas for compound libraries are currently being requested from the academic and industrial research community. Initial ideas have been provided from members of the consortium following strategies including navigation of biologically relevant chemical space; innovative library design; discovery of high quality chemical probes; and synthetic approaches that enable systematic exploration of chemical space3. It is also the aim of the ELF to provide an opportunity for scientists outside the consortium to contribute ideas, and this is now possible through a web-based tool on the ELF website (www.europeanleadfactory.eu/proposals/chemical-scaffolds/). All suggested ideas will be prioritized on the basis of their suitability for the JECC by being scored against a number of parameters including molecular properties, structural features, synthetic tractability, diversity potential, novelty and innovation. Selected proposals will then be validated to ensure that preparation of a library is practical. The syntheses of libraries will then be carried out at one of the five chemistry SMEs involved in the project - Edelris, Mercachem, Sygnature, Syncom and Tarosi4. Successful proposals will attract a financial reward. Overall, the aim is to provide 200,000 compounds using this approach, eventually bringing the JECC to approximately 500,000 compounds.
Compound Storage and Logistics
The JECC is a vital and valuable resource for the project. It is held in some of the most advanced compound management facilities in Europe, in particular the state-of-the-art compound store managed by BioAscent5 that is based on REMP technology. Compounds are stored in DMSO at -20°C under a controlled atmosphere and benefit from storage in single-use tubes, minimizing freeze thaw cycles and ensuring the maximum lifetime for the compounds. The REMP technology allows for rapid cherry-picking of samples, facilitating fast follow-up of interesting hits. Supporting facilities ensure samples can be provided in a flexible array of formats. Samples are provided to the European Screening centre for screening of public programs, and to the EFPIA partners for their screens and follow up.

Automated Compound Store at BioAscent, Newhouse
Public Targets
A key gap being addressed by the ELF is access to screening and follow-up for public targets. Target ideas suggested from outside the consortium are prioritized through a review process, and successful programs gain access to assay development, high throughput screening, hit characterization and medicinal chemistry. Targets are currently being sought and can be submitted via the website (www.europeanleadfactory.eu), where an application form and more details on terms and conditions can be found. Submitted targets are required to pass three key assessment procedures before practical work will begin. The first is to meet defined eligibility criteria. As innovation is a key goal of the project, it is not intended that the compound collection will be screened versus a target more than once. The first step is therefore to ensure that this criterion has been met and that the target owner is eligible for IMI funding. The second criterion is one of technical feasibility. Is the assay in a suitable format for screening using technology available to the screening centre? A full description of assay requirements is available on the website (www.europeanleadfactory.eu). The final criterion is one of prioritization. This is carried out by a committee of internal and external experts who assess the target idea for scientific quality, innovative potential, disease relevance and ‘fit’ within the portfolio. A broad range of disease areas is acceptable and once a target has passed these three stages and is accepted for practical work, a dialog between the screening centre and the target owner begins, ensuring that the expectations and aspirations of the target owner are fully understood and are the basis of the screening plan.
Screening
The screening centre is based around the two former Organon/MSD sites in Scotland and The Netherlands and utilizes key infrastructure designed for this purpose at those sites. In addition, resources are available at the Universities of Dundee and Oxford, providing specialist capabilities in protein production, biostructural studies, biophysics, DMPK and informatics. A wide selection of assay technologies are available at the screening centre including absorbance, fluorescent intensity, fluorescent polarization, homogenous time resolved fluorescence and alphascreen.
Ultra High Throughput screening takes place at the Pivot Park Screening Centre6 (PPSC), which provides automated cellular and biochemical in-vitro screening capability. To ensure that suggested assays can be migrated onto the robotics required for screening, a dedicated assay development team is available at PPSC and multiple high throughput screening platforms are available. Screening will be carried out in 1536 well format when scalable, but 384 well format will also be acceptable.

State-of-the-art screening robot at Pivot Park Screening Centre in Oss
Hit Characterisation and Medicinal Chemistry
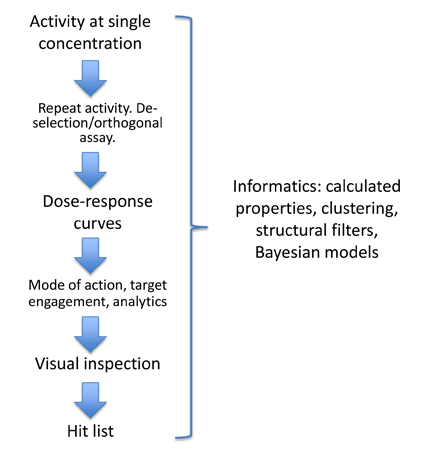
A key goal of the ELF is to provide the highest quality hit lists to support target owners in applications for further funding or attracting downstream collaborations and partners. The data package provided with the hit list will be tailored to the requirements of the program and will be used to answer key questions regarding the chemical matter identified in the screen e.g. is the hit compound interacting with the target in the way we expect?, do we know the composition of the active entity?, does the hit compound interact with other targets?, and, is the compound a good starting point for future work?
Data can include dose-response curves, selectivity information, evidence of target engagement, in vitro DMPK and analytical chemistry. The aim will be to initiate medicinal chemistry on some of the programs and thereby start to generate structure-activity relationships, identify liabilities with the hits and assess their scope for optimization. For a small number of programs there will be the opportunity to combine these data with xray crystallographic information.
This process will be supported by informatics tools allowing multiple calculated parameters to be included in the triage e.g. lipophilicity, QED score7. In addition clustering will enable prioritisation versus Bayesian models of a range of selectivity or anti-targets.
Informatics, Knowledge Management, Honest brokerage and Open Innovation
Another key goal of the ELF is to create a knowledge base and to review successful strategies for library design. There is an opportunity, created by screening the same collection against many targets, to measure library performance against multiple parameters including frequent hitter potential and hit rate versus target class. In silico models of bioactivity will be generated as well as an assessment of the effect on outcomes of screening of size and dimensionality of libraries. Machine learning models will assist in prioritization of compounds and in false-negative rescue.
With 30 members of the ELF consortium8 and potentially a much larger number of external collaborators exploring approaches to open innovation is a clear opportunity for the ELF. Implementing clear procedures to ensure the appropriate flow of information and protecting confidentiality is an absolute requirement and this has been established through the use an “Honest Data Broker” system which will provide valuable experience for other complex open innovation collaborations.
Conclusions
The availability of chemical matter to modulate novel biological targets is a key requirement for the discovery of novel pharmacological tools and for discovering new small molecule medicines and is a current gap in academic and SME translational research. Giving these research communities access to large pharma scale high throughput screening of high quality compound collections is a vital strategy for identifying that chemical matter and ensuring healthy long term drug development pipelines. To ensure that the quality of the screening output is worthy of further investment rigorous hit characterisation including medicinal chemistry is required. Through the funding provided by IMI, facilities and expertise to satisfy these requirements are now available to broad range of public and private partners. To get involved visit the website www.europeanleadfactory.eu
The ELF is funded with financial support from IMI JU Grant Agreement 115489
References:
- www.imi.europa.eu
- Big pharma screening collections: more of the same or unique libraries? The AstraZeneca-Bayer Pharma AG case. J Huser et al, Drug Discovery Today 2013, 18, 1014-1024.
- Biologically Active Macrocyclic Compounds – from Natural Products to Diversity-Oriented Synthesis. M.H. Clausen, Eur. J. Org. Chem. 2011, 3107-3115. Natural product-inspired cascade synthesis yields modulators of centrosome integrity. K. Kumar, H. Waldmann et al, Nat. Chem. Biol. 2012, 8, 179-184. Synthesis of Natural-Product-Like Scaffolds in Unprecedented Efficiency via a 12-Fold Branching Pathway, R. Stockman et al, Chemical Science, 2011, 2, 2232. A. Nelson, “Synthesis of natural product-like molecules with over eighty distinct skeletons”, Angew. Chem., Int. Ed. 2009, 47, 104-109.
- www.edelris.com, www.mercachem.com, www.sygnaturediscovery.com, www.syncom.nl, www.taros.de
- www.bioascent.com
- www.pivotparkscreeningcentre.com
- Quantifying the chemical beauty of drugs. Andrew L Hopkins et al Nature Chemistry (2012) 4(2), 90-98
- AstraZeneca AB, Bayer Pharma AG, BioAscent Ltd, ChemAxon Kft., Danmarks Tekniske Universitet, Edelris SAS, Foundation Top Institute Pharma, Gabo:mi GmbH, H. Lundbeck A/S, Janssen Pharmaceutica NV, Lead Discovery Center GmbH, Max Planck Institute Dortmund, Mercachem BV, Merck KGaA, Nederlands Kanker Instituut, Pivot Park Screening Centre BV, Radboud University Nijmegen, Rijksuniversiteit Groningen, Sanofi-Aventis GmbH, Sygnature Discovery Ltd., Syncom BV, Taros Chemicals GmbH, UCB Pharma SA, Universitat Duisburg-Essen, Universiteit Leiden, University of Dundee, University of Leeds, University of Nottingham, University of Oxford and VU University Medical Center Amsterdam.









