NiKem Research Srl

NiKem Research Srl (NiKem) started operations in 2001 as a spin-off from the SmithKline Beecham (GSK) Milan Discovery centre and is located at Baranzate (Mi, Italy); it is a specialized drug discovery company whose mission is to create value to the Pharmaceutical Industry capitalizing on the over 20-year average experience in medicinal chemistry and drug discovery cumulated by its Management Team within a multinational company.
NiKem has an international reputation as a strategic and consolidated partner on the basis of more than ten years of operations in Pharmaceutical Research and Development offering a wide range of services, such as synthetic and medicinal chemistry, biochemical pharmacology, in vitro ADMET, in vivo DMPK and preliminary rodent toxicology, preclinical GLP and clinical GCP bioanalysis. All these modules can be sold as an integrated platform or as standalone services, thus allowing great flexibility to the business model and great compliance with client’s needs.
To fully realize its value proposition, NiKem implemented and developed an appropriate infrastructure in terms of facilities, technology platform and equipments, all instrumental to offer a demonstrated advantage to its clients. Making the best use of this technology platform, NiKem scientists are able to offer an added value output obtained in the shortest possible timeframe with a significant return on investment for the outsourcing company.
The hidden gem inside NiKem is actually its staff, a balanced mix of synthetic and medicinal chemists, biologists and biotechnologists, most of them with more than 10 years of experience in the pharmaceutical sector, very well coordinated and supervised by the Management Team with over 25 years in average of experience in drug discovery. In view of the increased competition in the outsourcing drug discovery arena, it is not enough to have well trained people and best scientific competence and technologies. NiKem is fully oriented to understand and satisfy the different needs of its clients, implementing customer-tailored solutions and adopting a client-oriented flexibility. This means that NiKem not only has a well trained and experienced staff but also that these people are able to work together in a “team approach model”. Given the multidisciplinary environment of the drug discovery & development process this is a great plus as NiKem teams perfectly know “when” a certain phase or issue will come during the process and “how” it should be solved.
To boost the competitiveness of the company, at the end of 2007 NiKem implemented a 3-year strategic industrial development plan to integrate the technologies and skills already existing on site with new emerging technologies and certifications:
- MALDI/TOF/TOF (Mass Spectrometry time of flight) for ex vivo ‘imaging’ studies;
- Expansion of services within rodents in vivo treatments (pharmacokinetics, toxicokinetics, PK/PD, and in vivo pharmacology);
- Achievement of Good Laboratory Practice (GLP) certification for pharmacokinetic and metabolism (Certificate of Compliance to the Good Laboratory Practice, granted from the Ministry of Health in 2009);
- Achievement of authorization to perform rodent acute and sub-chronic toxicology and toxicokinetic studies (Authorization by Ministry of Health Decree in 2010);
- Achievement of qualification and subsequent authorization in 2010-2011 from regulatory authorities (AIFA – Italian Agency for Medicines) to perform pharmacokinetic, metabolism and bioequivalence analyses on human samples (plasma, blood, urine, etc.) coming from Phase 1-3 clinical studies.
The excellence of NiKem staff is testified by the 54 patents filed in 11 years (NiKem scientists as inventors and the various clients as assignees) and by the 66 articles published on top international journals on various aspects of medicinal and synthetic chemistry, biochemical pharmacology, pharmacokinetic and drug discovery strategy. From its inception, NiKem has conducted more than 80 medicinal chemistry projects for its clients and more than 100 custom synthesis projects, including 12 library syntheses for either big or mid-size pharmaceutical or small biotechnology companies. Within the medicinal chemistry projects, 11 compounds were approved, by specific Strategic Development Review Board internal to clients’ corporate organizations, for progression to full Pre-Clinical Development and 7 of these are today in Clinical Trials (Phase 1 or 2).
With reference to their mechanism of action, the medicinal chemistry projects can be subdivided as follows:
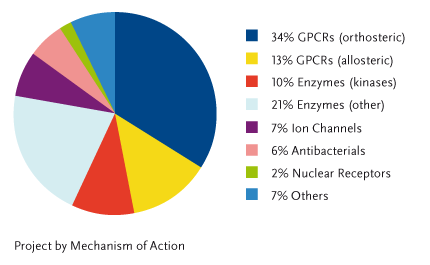
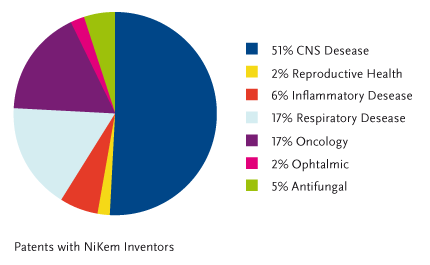
Instead, with reference to the patents filed by NiKem inventors, the following therapeutic areas of primary unmet medical need, are covered:
_____________________________________________________
Contact
NiKem Research
Website: www.nikemresearch.com
Email: info@nikemresearch.com
By Giuseppe Giardina, CEO & Managing Director









