|
|

Herbert Waldmann |
LAB PRESENTATION : Department of Chemical Biology
Max Planck Institute of Molecular Physiology
TU Dortmund

The department
The department of Chemical Biology is one of four departments at the Max Planck Institute of Molecular Physiology. Herbert Waldmann is leading this department since 1999, when he became director at the institute. He simultaneously holds a position as full professor for Bioorganic Chemistry at the TU Dortmund. In practice, both departments are linked closely, i.e. formal aspects are separated clearly, but daily work employs a joint research environment. Furthermore, there is a very close collaboration with the Chemical Genomics Center of the Max Planck Society, where chemists, biochemists and biologists from several Max Planck Institutes as well as Max Planck- and industry funded research groups are working under one roof to identify and develop new approaches to medicinal chemistry and chemical biology research.
Research in the Department of Chemical Biology is focused on the interface of organic chemistry and biology. New synthesis methods and strategies are developed and employed for the synthesis of compounds which then are used as probes for the study of biological phenomena.
 Interplay between Organic Synthesis and Biology in Chemical Biology.
Interplay between Organic Synthesis and Biology in Chemical Biology.
The synthesis and chemistry of proteins, in particular with a view to biological signaling and vesicular trafficking are major areas of activity at the Department. These activities are complemented by very recent research efforts aimed at the development of new methods for the making of protein arrays. Also the Department has intense activities in small molecule development for chemical biology research in particular based on natural products and compound collections derived therefrom.
These activities include the establishment of screening capacity employing both isolated proteins and cell-based screens and the identification of cellular target proteins, the development of new phenotype-based screening methods and incorporation of research programs employing bioinformatics and cheminformatics methods in order to guide our synthesis programs.
Head of the group
Herbert Waldmann studied chemistry at and graduated from the University of Mainz with a Ph. D. in organic chemistry in 1985 under the guidance of Horst Kunz. After a postdoctoral appointment with George Whitesides at Harvard University, he completed his habilitation at the University of Mainz in 1991. In 1991 he was appointed as professor of organic chemistry at the University of Bonn, and in 1993 he was appointed to full professor of organic chemistry at the University of Karlsruhe. In 1999 he accepted appointments as Director of the Max-Planck-Institute of Molecular Physiology Dortmund, and as Professor of Organic Chemistry at the University of Dortmund.
Research Focus
Chemical Biology of Protein Lipidation
Lipid-modified proteins play important roles in particular in biological signaling and vesicular transport. The corresponding research projects of the Waldmann group focus on the development of methods for the synthesis of characteristic lipid-modified peptides and entire functional lipidated proteins by means of a combination of organic synthesis and molecular biology techniques. Such semi-synthetic proteins then are employed in biochemical, biophysical, cell-biological and structural biology investigations yielding new insights into the biological phenomena the parent lipidated proteins are involved in.
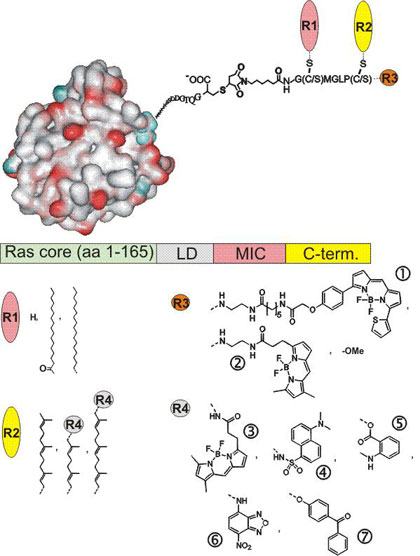 Collection of semisynthetic Ras proteins.
Collection of semisynthetic Ras proteins.
Biology oriented synthesis (BIOS)
The structural scaffolds of natural product (NP) classes are endowed with relevance to nature and provide evolutionarily selected starting points in chemical structure space for compound collection design and development. Since they emerge via biosynthesis by proteins and fulfill multiple functions via interaction with proteins, NP classes encode structural properties required for binding to these biomacromolecules. Biology oriented synthesis (BIOS) builds on these arguments and employs core structures delineated from natural products as scaffolds of compound collections.
Some examples for the synthesis of in-house libraries are: spiroacetals, Ñ,Ò-unsaturated lactones, tetrahydropyrans, oxepanes, indole derived compounds, indoloquinolizidines, decalines, and biarylpeptides.
Structural Classification of Ligand Binding Protein Cores and Natural Products
For the development of small molecules for chemical biology and medicinal chemistry research relevance in nature is the decisive criterion. Current research in the Waldmann group aims at the identification of biologically relevant and pre-validated starting points in vast structure space for compound collection development. In order to achieve this goal structural similarities in the ligand sensing cores of proteins and in their natural ligands, i.e. the small natural products emerging by biosynthesis are identified and used for similarity clustering and structural classification. This approach leads to hypothesis-generating tools setting the starting points for chemical genomics research, i.e. the identification and use of small molecules to elucidate the biological function of protein families. Due to this guidance by nature, the application of Protein structure similarity clustering (PSSC) and Structural classification of natural products (SCONP) in biology oriented synthesis (BIOS), should yield new opportunities for the discovery of unprecedented protein ligand classes with high hit rates at comparably small library size.
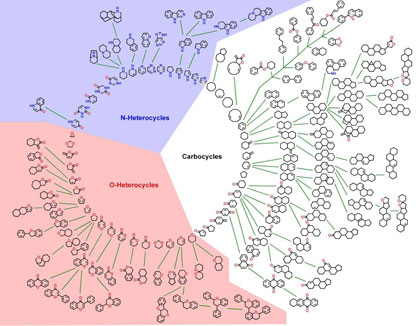 Tree-like structural classification of natural product scaffolds. The "NP tree" is shown at a resolution of 0.2% which means that scaffolds have to represent at least 200 structures in order to be displayed.
Tree-like structural classification of natural product scaffolds. The "NP tree" is shown at a resolution of 0.2% which means that scaffolds have to represent at least 200 structures in order to be displayed.
Biochemical and Cell-based Compound Screening
For the development of new tool compounds for chemical biology and medicinal chemistry research, the natural product inspired compound collections synthesized in the Department are screened in automated biochemical and cell based assays. The compound classes identified thereby are then employed as starting points for identification and validation of protein targets. New in vitro screens of biological interest as well as further cell based assays are developed in house and with external collaboration partners.
Examples for in house screens are: phosphatases, APT1, RabGGTase, reporter gene assays related to the Ras- and WNT-pathways, and phenotype based approaches relating to the Ras- and WNT-pathways and mitosis.
Teaching
The department is involved in bachelor and master programs for chemical biology and chemistry at the TU Dortmund. Mainly, it is responsible for the bioorganic topics in these programs. Weekly lectures about bioorganic chemistry are given at different levels for bachelor and master students.
The department hosts a practical course in Chemical Biology for bachelor and master students (see this link and Waldmann, Janning: Chemical Biology A Practical Course, Wiley-VCH, Weinheim 2004).
In regular in house progress reports PhD students and postdocs present their research to the whole department to discuss and develop further research strategies. Institute seminars given by invited experts provide a wide spectrum of views on current cutting-edge science of relevance to the research activities of the department.
People
In addition to the Department head currently ten group leaders and senior scientists are integrated into the research activities of the Department of Chemical Biology: Hans-Dieter Arndt, Lucas Brunsveld, Bruno Bulic, Christian Hedberg, Katja Hübel, Petra Janning, Markus Kaiser, Kamal Kumar, Heino Prinz and Daniel Rauh. For their research programs see: www.mpi-dortmund.mpg.de/forschungProjekte/AGs/AGAbtIV/index.html.
The department is scientific home to numerous senior scientists, postdocs, PhD and bachelor students, technicians, and trainees.
Selected publications
- Köhn M, Gutierrez-Rodriguez M, Jonkheijm P, Wetzel S, Wacker R, Schroeder H, Prinz H, Niemeyer CM, Breinbauer R, Szedlacsek SE, Waldmann H. A microarray strategy for mapping the substrate specificity of protein tyrosine phosphatase. Angew. Chem. Int. Ed. 46(40):7700-7703, 2007.
- Gelb MH, Brunsveld L, Hrycyna CA, Michaelis S, Tamanoi F, Van Voorhis WC, Waldmann H. Therapeutic intervention based on protein prenylation and associated modifications. Nature Chemical Biology. 2(10):518-528, 2006.
- Nören-Müller A, Reis-Correa I, Prinz H, Rosenbaum C, Saxena K, Schwalbe HJ, Vestweber D, Cagna G, Schunk S, Schwarz O, Schiewe H, Waldmann H. Discovery of protein phosphatase inhibitor classes by biology-oriented synthesis. Proc. Natl. Ac. Sci. 103(28):10606-10611, 2006.
- Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PIH. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 307(5716):1746-1752, 2005.
- Koch MA, Schuffenhauer A, Scheck M, Wetzel S, Casaulta M, Odermatt A, Ertl P, Waldmann H. Charting biologically relevant chemical space: A structural classification of natural products (SCONP). Proc. Natl. Ac. Sci. 102(48):17272-17277, 2005.
- Koch MA, Wittenberg LO, Basu S, Jeyaraj DA, Gourzoulidou E, Reinecke K, Odermatt A, Waldmann H. Compound library development guided by protein structure similarity clustering and natural product structure. Proc. Natl. Ac. Sci. 101(48):16721-16726, 2004.
- Rak A, Pylypenko O, Durek T, Watzke A, Kushnir S, Brunsveld L, Waldmann H, Goody RS, Alexandrov K. Structure of Rab GDP-dissociation inhibitor in complex with prenylated YPT1 GTPase. Science. 302(5645):646-650, 2003.
By Petra Janning
|
|

Editor
Gerhard Ecker
Univ. Vienna, AT
Editorial Committee
Koen Augustyns
Univ. Antwerpen, BE
Erden Banoglu
Gazi Univ., TR
Gabriele Costantino
Univ. Parma, IT
Jordi Mestres
IMIM-UPF, ES
Kristian Stromgaard
Univ. Copenhagen, DK

Executive Committee
Roberto Pellicciari President
Gerhard F.Ecker Pres. Elect
Rasmus P.Clausen Secr/Treas
David Alker Member
Brigitte Lesur Member
Peter Matuys Member
|







