|
|

Roberto Pelliciari |
LAB PRESENTATION
The Pellicciari Group, Department of Chemistry and Drug Technologies, University of Perugia
The Medicinal Chemistry research group at the Department of Chemistry and Drug Technologies of the University of Perugia (Italy), led by Roberto Pellicciari, is dedicated to the effective and efficient use of knowledge in investigating new reactions, design and synthesize bioactive molecules, and in devising new analytical procedures.
The Research Group
The group is structured in four highly complementary and integrated research areas including (i) Medicinal Chemistry, (ii) Organic Synthesis, (iii) Molecular Modelling, and (iv) Pharmaceutical Analysis. Focussing on this multi-disciplinary approach, the research activities of Pellicciari group are centred on the design and synthesis of biologically active molecules for nuclear receptors, GPCRs, and a variety of enzymes as main therapeutic targets in CNS disorders, cancer, liver and metabolic diseases. The compounds employed as chemical tools are the basis for the biological investigations carried out with several international collaborations.
Via research in both organic and medicinal chemistry, the group has synthesised more than 2000 final compounds, including natural products, semi-synthetic derivatives, and small molecules with drug-like properties across a number of target classes and diseases that are highlighted below.
Key Achievements and Main Areas of Research
Selective Modulators of Genomic and Non-Genomic Bile Acid Signalling Pathways
Bile acids have recently been rediscovered as key elements of paracrine and endocrine functions related to the homeostasis of cholesterol levels, control of lipid and carbohydrate metabolism, and regulation of the immune system. These effects are mediated by the activation of two distinct pathways: genomic signalling (transcriptional control), mediated by the activation of the farnesoid X nuclear receptor (FXR), and non-genomic cell signalling activated by TGR5, a G-protein-coupled receptor.
Extensive investigation of the chemistry of bile acids has provided insights into the conformational and structural features that control their selective activation of genomic versus non-genomic effects, and allowed the design and synthesis of novel bile acid analogues that are able to pharmacologically differentiate between these effects (Figure 1).
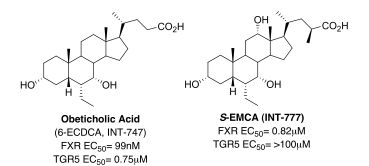
Figure 1. Switching bile acid selectivity
Moreover, the development of new synthetic methodologies for the structural modification and functionalization of bile acids are crucial not only for the synthesis of new derivatives with improved ADMET and PK/PD profiles, but also for the large scale production of these therapeutically active bile acid derivatives. We have also focused on physico-chemical profiles, for example, the critical micellar concentration (CMC) where the chromatographic index ϕ0 serves to indicate the propensity of bile acid derivatives to form micelles, and has been shown to be instrumental for the evaluation of the hydrophobic/hydrophilic balance of bile acids.
Culminating from this combined expertise, Obeticholic Acid (6-ECDCA, INT-747), a first-in-class FXR agonist, was discovered and is now completing Phase II clinical trials, with Intercept Pharmaceuticals, for the treatment of primary biliary cirrhosis (PBC) and non-alcoholic steatohepatitis (NASH). Obeticholic acid is an analogue of the primary human bile acid chenodeoxycholic acid, the endogenous FXR agonist, and is based on the observation that FXR agonist activity is strongly increased when a small alkyl group, in particular, an ethyl group is introduced at the 6α-position of chenodeoxycholic acid. Positive Phase II results (2009) from studies in type-2 diabetes with non-alcoholic fatty acid disease (NAFLD) and in patients with refractory primary biliary cirrhosis (PBC), support obeticholic acid’s potential as a novel, hepatoprotective agent in a broad range of chronic liver diseases. Currently, obeticholic acid is planned to proceed to Phase III trials for PBC, while pursuing additional studies in other indications such as, NASH and portal hypertension.
Building on the group’s knowledge of bile acids and in particular cholic acids, a potent, selective, orally bioavailable TGR5 agonist, S-EMCA (INT-777) derived from the incorporation of a methyl and ethyl moiety respectively at the C23(S)- and C6α-positions was developed. In preclinical models of obesity, S-EMCA increases basal energy expenditure in diet-induced obese mice; prevents weight gain and adiposity; induces GLP-1 secretion and insulin sensitivity, and normalizes glycemic control. In high fat fed mice it reduces lipid levels as well as liver steatosis and fibrosis. S-EMCA and analogue thereof are currently being advanced for use in obesity and type-2 diabetes.
Selected publications :
- 1.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6Alpha-ethyl-chenodeoxycholic acid (6-ECDCA),a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem. 2002, 45, 3569-3572.
- 2.Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, Fiorucci S, Clerici C, Gioiello A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J. Med. Chem. 2004, 47, 4559-4569.
- 3.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008, 7, 678-693.
- 4.Sato H, Macchiarulo A, Thomas C, Gioiello A, Une M, Hofmann AF, Saladin R, Schoonjans K, Pellicciari R, Auwerx J. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J. Med. Chem. 2008, 51, 1831-1841.
- 5.Pellicciari R, Gioiello A, Macchiarulo A, Thomas C, Rosatelli E, Natalini B, Sardella R, Pruzanski M, Roda A, Pastorini E, Schoonjans K, Auwerx J. Discovery of 6α-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 2009, 52, 7958-7961.
- 6.Natalini B, Sardella R, Camaioni E, Macchiarulo A, Gioiello A, Carbone G, Pellicciari R. Derived chromatographic indices as effective tools to study the self-aggregation process of bile acids. J. Pharm. Biomed. Anal. 2009, 50, 613-621.
Modulators of Excitatory Glutamatergic Pathways
Neurodegenerative and neuroprotection processes are driven by highly complex and plastic signalling networks. In order to be able to generate insights into the physiological and pathological role of diverse targets within these signalling networks the development of approaches to the design and synthesis of new chemical entities capable of modulating specific enzymes and receptors is required. The synthesis of conformational constrained analogues of L-glutamic acid (Figure 2), for example, provided potent and selective chemical tools and has allowed the characterization of glutamatergic signalling pathways in the central nervous system and investigation of their role in neurodegenerative and neuroprotection processes.
The Pellicciari group has a long standing interest in the complex medicinal chemistry of L-glutamic acid (L-Glu), the main human excitatory neurotransmitter, addressing the design and synthesis of non-proteinogenic amino acid derived modulators of ionotropic and metabotropic glutamate receptors. For example, conformational freezing of the carbon skeleton through ring insertion and bioisosteric replacement of the distal carboxylic group, led to several classes of potent and selective glutamatergic modulators (Figure 2).
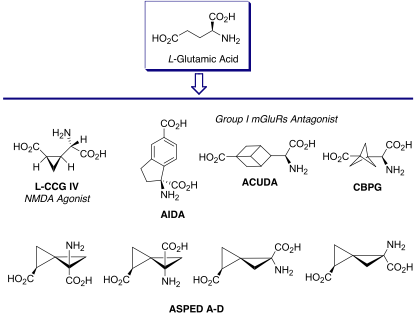
Figure 2. ‘Frozen’ glutamate analogues as excitatory amino acid receptor ligands
Selected publications :
- 1.Pellicciari R, Marinozzi M, Camaioni E, del Carmen Nùnez M, Costantino G, Gasparini F, Giorgi G, Macchiarulo A, Subramanian N. Spiro[2.2] pentane as a dissymmetric scaffold for conformationally constrained analogues of glutamic acid: focus on racemic 1-aminospiro[2.2]pentyl-1,4-dicarboxylic acids. J. Org. Chem. 2002, 67, 5497-5507.
- 2.Pellicciari R, Marinozzi M, Macchiarulo A, Fulco MC, Gafarova J, Serpi M, Giorgi G, Nielsen S, Thomsen C. Synthesis, molecular modeling studies, and preliminary pharmacological characterization of all possible 2-(2'-sulfonocyclopropyl)glycine stereoisomers as conformationally constrained L-homocysteic acid analogs. J. Med. Chem. 2007, 50, 4630-4641.
Interest in DNA damage repair mechanisms arising from hyperactivation of glutamate receptors led to an interest in poly (ADP-ribose)polymerases (PARPs).
Selective Inhibitors of Poly (ADP-ribose) polymerases
PARP uses NAD+ as a substrate and poly(ADP-ribos)ylates a variety of proteins, including histones, caspases, topoisomerases, and PARP itself. In the presence of moderate DNA damaging, poly(ADP-ribosyl)ation is a covalent modification which allows the enzymatic machinery to repair and maintain the genomic integrity of cells including injured neurons. In the presence of severe damage, such as those resulting from acute brain insult, the genomic integrity cannot be preserved and PARP-1 executes a death signal by consuming NAD, depleting ATP stores, and causing an energy crisis in neurons. The group has developed a novel series of thieno[2,3-c]isoquinolin-5(4H)-one PARP inhibitors (Figure 3) via a modelling and rational drug design approach leading to highly potent compounds such as HYDAMTIQ (Ki=29nM PARP-1/2) efficacious in both in vitro and in vivo models of ischemia.
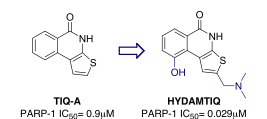
Figure 3.
Selected publications :
- 1.Costantino G, Macchiarulo A, Camaioni E, Pellicciari R. Modeling of poly(ADP-ribose)polymerase (PARP) inhibitors. Docking of ligands and quantitative structure-activity relationship analysis. J. Med. Chem. 2001, 44, 3786-3794.
- 2.Bellocchi D, Macchiarulo A, Costantino G, Pellicciari R. Docking studies on PARP-1 inhibitors: Insights into the role of a binding pocket water molecule. Bioorg. Med. Chem. 2005, 13, 1151-1157.
- 3.Pellicciari R, Camaioni E, Gilbert AM, Macchiarulo A, Bikker J, Costantino G, Gioiello A, Robertson GM, Sabbatini P, Venturoni F, Liscio P, Carotti A, Bellocchi D, Cozzi A, Wood A, Gonzales C, Zaleska MM, Ellingboe J, Moroni F. Discovery and Characterization of Novel Potent PARP-1 Inhibitors Endowed with Neuroprotective Properties: From TIQ-A to HYDAMTIQ. J. Med. Chem. In press.
Diazo Chemistry
Diazo compounds have found numerous applications in organic chemistry because of their versatility in several synthetically useful transformations making them valuable precursors and intermediates of biologically active compounds (Figure 4). Diazo chemistry is illustrative of the creativity and patience needed to acquire chemical control on reagents. Particular attention has been devoted in exploring the α-diazocarbonyl compounds as a source of carbenoids, and in the study of diverse reactivity of diazo substrates prepared from the reaction of cyclic and acyclic ketones, and aldehydes with ethyl diazoacetate, diazoacetone, and ethyl pyruvate by using different sets of reaction conditions. The results, have not only been instrumental for the development of new synthetic methodologies, but have also provided new insights into the mechanistic aspects of the reactions as well as an understanding of the factors and experimental conditions which govern the products formed and their relative distribution.
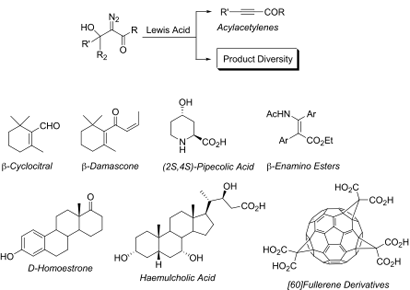
Figure 4.
Selected publications :
- 1.Pellicciari R, Frenguelli R, Sisani E. A new synthesis of β-damascone. Tetrahedron Lett. 1980, 21, 4039-4042.
- 2.Pellicciari R, Natalini B, Frenguelli R. An efficient procedure for the regiospecific preparation of d-homo-steroid derivatives. Steroids 1987, 49, 433-441.
- 3.Pellicciari R, Natalini B, Sadeghpour BM, Marinozzi M, Snyder JP, Williamson BL, Kuethe JT, Padwa A. The Reaction of α-Diazo-β-hydroxy Esters with Boron Trifluoride Etherate:Generation and Rearrangement of Destabilized Vinyl Cations. A Detailed Experimental and Theoretical Study. J. Am. Chem. Soc. 1996, 118, 1-12.
- 4.Gioiello A, Venturoni F, Natalini B, Pellicciari R. BF3.Et2O-induced decomposition of ethyl 2-diazo-3-hydroxy-3,3-diarylpropanoates in acetonitrile: a novel approach to 2,3-diaryl beta-enamino ester derivatives. J. Org. Chem. 2009, 74, 3520-3523.
The “Other Side” of Chemical Space
Recent years have seen a growing interest in exploration and navigation of the chemical space defined by the set of all possible small molecules. Accordingly, diverse strategies have been developed to explore and identify the limited portions of the chemical space where biologically active molecules lie. Our interest has been in the exploration of the “other side” of chemical space, that is, the biological counterpart of chemical space which is composed of the ensemble of protein structure binding sites. Charting the “other side” of chemical space provides a biological knowledge which when combined with chemical knowledge, aids insights into the principles that govern molecular recognition and bioisosteric relationships of functional groups. For example, applications of navigating the “other side” of chemical space have been pursued as a more effective strategy for studying the bioisosteric relationships of acidic and basic groups (Figure 5).
Figure 5. Amine Binding Sites Selected at the Edges of the Chemical Space of Target Site
Selected publications :
- 1.Macchiarulo A, Nuti R, Eren G, Pellicciari R. Charting the chemical space of target sites: insights into the binding modes of amine and amidine groups. J. Chem. Inf. Model. 2009, 49, 900-912.
- 2.Macchiarulo A, Pellicciari R. Exploring the other side of biologically relevant chemical space: insights into carboxylic, sulfonic and phosphonic acid bioisosteric relationships. J. Mol. Graph. Model. 2007, 26, 728-739.
Head of the Group
 Professor Roberto Pellicciari initiated his scientific career working on natural products at the Istituto Superiore di Sanità and then at the Universidad de Carabobo (Venezuela) as Visiting Professor (1968-1970). From 1970 to 1973, he was Research Associate with Prof. Ernest Wenkert at the Department of Chemistry at Indiana University, Bloomington (USA) and Visiting Professor in the Department of Chemistry, University of California at San Diego (UCSD), La Jolla (1980-1981). He is currently full Professor of Medicinal Chemistry at the Department of Chemistry and Drug Technologies (University of Perugia) and adjunct Professor at the Department of Psychiatry, School of Medicine University (Maryland, USA). He is also a co-founder and Head of Medicinal Chemistry at Intercept Pharmaceuticals (New York, USA). Professor Roberto Pellicciari initiated his scientific career working on natural products at the Istituto Superiore di Sanità and then at the Universidad de Carabobo (Venezuela) as Visiting Professor (1968-1970). From 1970 to 1973, he was Research Associate with Prof. Ernest Wenkert at the Department of Chemistry at Indiana University, Bloomington (USA) and Visiting Professor in the Department of Chemistry, University of California at San Diego (UCSD), La Jolla (1980-1981). He is currently full Professor of Medicinal Chemistry at the Department of Chemistry and Drug Technologies (University of Perugia) and adjunct Professor at the Department of Psychiatry, School of Medicine University (Maryland, USA). He is also a co-founder and Head of Medicinal Chemistry at Intercept Pharmaceuticals (New York, USA).
Professor Pellicciari enjoys an international reputation in molecular design, synthesis, mechanism of reactions, and modelling studies with more than 300 publications in international journals and 40 patents. His main research fields are; the design and synthesis of novel biologically active molecules in particular for nuclear and GPCR receptors of CNS and metabolic pathways, and the development of robust knowledge-based design and medicinal chemistry methodologies.
Prof. Pellicciari has been awarded of several prizes such as the “Domenico Marotta” prize of the “Accademia Nazionale delle Scienze” (1999), the “Mentzer” prize of the French “Société de Chimie Thérapeutique” (2001), the “Giacomello Medal” of the “Divisione di Chimica Farmaceutica (SCI)” (2006) and the “Amedeo Avogadro Medal” of the Italian Chemical Society (SCI) (2009).
Professor Pellicciari has served on many national and international committees, including; Director of the Advanced School of Medicinal Chemistry of the Italian Chemical Society and President of the Division of Medicinal Chemistry of the Italian Chemical Society (2001-2003), and President of the European Federation of Medicinal Chemistry, EFMC (2006-2008).
Organic Synthesis
In a time in which organic synthesis moves beyond its traditional objectives and major changes are introduced in the choice of synthetic targets, the area of ‘Synthetic Methods Development’ continue to be an issue of great impact. Having the role to define the strategies and to provide the tools within which new target molecules can be reached, organic synthesis is an obliged crossway for disciplines such as medicinal chemistry, and an effective means for the discovery of new reactions or the uncovering of new aspects of previously described ones. In Pellicciari group, Maura Marinozzi, Emidio Camaioni and Antimo Gioiello put these aims into practice. In particular, research efforts are constantly directed to the study of novel reactions and relative applications and, more in general, to the development of novel methodologies key to the synthesis of biologically active compounds.
Molecular Modelling
The Pellicciari’s group was among the firsts in Italy to create an integrated environment between synthetic medicinal chemistry and molecular modelling. In 1994 the first lab of molecular modelling was installed in the group under the responsibility of Gabriele Costantino, at that time assistant professor and now professor of medicinal chemistry in Parma. The group has constantly grown up during the years and is now headed by Antonio Macchiarulo, with Andrea Carotti in the staff, and several graduate and post-graduate students completing a very productive environment which is continuously attracting students and visiting researchers also from abroad.
The aim of the lab is to provide constant computational support to the group’s main activities and, at the same time, to spin off exploratory projects which could eventually be integrated in the group’s platform.
Pharmaceutical Analysis
More recently, a Pharmaceutical Analysis Section has been implemented in the group under the supervision of Prof. Benedetto Natalini, with Roccaldo Sardella in the staff. Besides the analytical control in support of the organic synthesis, two main topics have been developed and are currently the subject matter of research. The former consists in the study of the mechanistic aspects and applications of new chiral selectors in the Chiral Ligand-Exchange Chromatography of amino acids. The latter is focused on the development and application of derived chromatographic indices in the physico-chemical profiling of new synthetic bile acids.

People
The Pellicciari Group comprises one full professor, Benedetto Natalini, three associate professors, Maura Marinozzi, Emidio Camaioni, and Antonio Macchiarulo, and three researchers, Antimo Gioiello, Andrea Carotti, Roccaldo Sardella. Overall, the units currently employ ca. 4 post docs, ca. 5 PhD students, and ca. 2 research assistants with, 10-15 undergraduate students researching their thesis projects each year.
Past-members of the Pellicciari group include; Gabriele Costantino, who moved to the University of Parma, in 2007, as full professor in Medicinal Chemistry at the Department of Pharmaceutics. Whilst Graeme Robertson recently joined the group as Research Professor.
Contact
Professor Roberto Pellicciari
Dipartimento di Chimica e Tecnologie del Farmaco
Università di Perugia
via del Liceo 1 06123 Perugia Italy
E-mail: rp@unipg.it
|
|

Editor
Gabriele Costantino
Univ. of Parma, IT
Editorial Committee
Erden Banoglu
Gazi Univ., TR
Leonardo Scapozza
Univ. of Geneve, CH
Wolfgang Sippl
Univ. Halle-Wittenberg, DE
Sarah Skerratt
Pfizer, Sandwich

Executive Committee
Gerhard F. Ecker President
Roberto Pellicciari Past Pres.
Koen Augustyns Secretary
Rasmus P. Clausen Treasurer
Javier Fernandez Member
Mark Bunnage Member
Peter Matuys Member

|









