
Big Pharma is in big trouble: over 200.000 jobs were already lost in the past decade.
When you are reading these numbers it does not really touch you, unless you are one of them. This is what happened to the researchers working in Weesp, the Netherlands in the former Solvay Pharmaceuticals laboratories. After the take-over by Abbott they heard the message in September 2010: all Research in Weesp will be stopped per April 1st 2011.
The management of the medicinal chemistry department (Chris Kruse and Bart van Steen) decided to start initiatives to safeguard the unique scientific know-how that belonged to the members of their research unit. Their collaborators have worked with great passion in the area of industrial drug discovery research for a large number years and it would be most regretful if this experience would get lost.
In shaping our new future we considered that the changing environment forces big Pharma companies to find other ways to reach their goals. Collaboration with academia as well as with small Biotech companies and with CRO’s has become the new philosophy. Clearly this state of affairs should give us new opportunities. After comprehensive studies and numerous discussions, also with Abbott Healthcare Products, we decided that our chance of success would be highest if we would establish a novel CRO in the area of multidisciplinary lead optimization. This focus was clearly seen as relevant for both big Pharma and for Biotech companies and represented a relatively unexplored niche amongst the present CRO’s.
Next we looked for professional help, because setting-up and running a company was not our speciality. We were fortunate to find two new colleagues (Herman Helder and Melchior van Voorden) having experience in setting up new businesses in the Pharma field. The NIBC supported us by conducting all financial assessments.

The result was that we started our new company Pharma Plexus Holland in August 2011.
We would present ourselves as “your experienced partner in lead optimization being able to accelerate your preclinical research projects”. This claim may sound somewhat bold, but we were convinced that it was justified based upon the long-standing experience of Pharma Plexus Holland staff with on average over 20 years in multidisciplinary lead optimization. This includes the central nervous system, recognized as the most difficult area in lead optimization. Our team has accomplished the completion of many drug discovery projects, leading to 15 clinical candidates in less than 10 years!
Quickly we could start with our first projects and we were able to offer a job to ca 20 people. They were delighted to be able to continue with their passion: turning hits and leads into molecules with optimized properties to advance into clinical trials. We started working in our former laboratories, but quickly it became clear that moving to a Science Park environment was business-wise more beneficial for Pharma Plexus Holland. With the help of Abbott we settled our company in the Utrecht Science Park, where we could move into the former organic chemistry laboratories of Utrecht University.

After one year our dreams have become reality. A customer recently evaluated our activities with the following statements:
- “The Pharma Plexus team is composed of and led by true medicinal chemists and differentiates itself from other chemistry specific teams in their ability to contribute to design of targets. Moreover, PPH may be characterized by high level of synthetic chemistry skill, genuine scientific curiosity and passion for drug discovery.”
- “Expectations were high with this team and yet they exceeded in this area. The team possesses a high level of professionalism and made excellent use of the combined years of experience to deliver high impact novel molecules. Open communication and commitment to tasks made for a highly successful collaboration.”
- “This was not a routine project and Pharma Plexus succeeded where multiple other pharmaceutical teams have failed. This was in part due to the willingness of the team to focus on expanding our SAR understanding through preparation of impactful molecules rather than focusing on libraries of a single functional handle.”
Our customer honoured Pharma Plexus Holland with their award of “best external company”. During the awarding ceremony the client summarized the performance by the words: “Pharma Plexus succeeded to design the right compounds and to synthesize these novel complex molecules with an unprecedented high speed!”
 What are the ingredients of innovative lead optimization and what is the basis of our success?
What are the ingredients of innovative lead optimization and what is the basis of our success?
The current new chemical entities that are approved as drugs for the pharmaceutical market need to fulfil many requirements. Consequently, present drug discovery research requires increasing innovation, especially during the early stages. Current lead optimization approaches include the subtle interplay of using and optimizing the right molecular properties. Actually, lead optimization is just like managing the well-known Rubik’s cube which aims to find simultaneously the right colour combinations in all different directions.
In our experience, the exchange of information between molecular biologists, medicinal chemists and pharmacologists is a key feature to run a drug discovery process efficiently and to meet all requirements of a drug candidate. Close interaction between experts will result in the ability to tackle the right problems at the right point in time.
An integrated and multidisciplinary approach requires mobilizing a professional team of medicinal chemists, including designers and synthetically as well as analytically skilled experts, and stimulating strong cooperation between these disciplines. Each expert has his/her own specialism, but they all understand each other; they all speak the language of potential drug-like molecules. In practice we follow a fully integrated approach where all aspects of medicinal chemistry and drug optimization perfectly link up with each other: studying existing knowledge, interpreting produced data, determining relevant parameters, drawing up optimization schemes, designing molecular structures, synthesizing compounds and evaluating their pharmacodynamic as well as their pharmacokinetic potential.

Each optimization project requires a tailored approach with its own dynamic flow chart, comprising an optimizing schedule that may consist of several different parameters to be optimized based on the available information. Our experienced medicinal chemists know what pitfalls can be expected during the optimizing project. They are able to adjust their strategy and to avoid the wrong choices.
Except of the well-known pharmacodynamic parameters such as target affinity, target activity and target selectivity, which often are given high priority, there are numerous other parameters that are at least as important. For instance intrinsic physicochemical properties such as solubility or lipophilicity and pharmacokinetic issues like metabolic stability, membrane permeability and the presence of free fractions in plasma and brain. In practice, however, optimization of these many different parameters may lead to contradictory demands to the molecular structure.
An essential element for any candidate drug is to secure the intellectual property through patenting. It plays a vital role already at the start of lead optimization. Our scientists with extensive experience in the pharmaceutical industry are well skilled in this field to understand the importance of patentability and to advise about optimal timing of patent applications. Moreover we as a CRO do not claim any intellectual property: all the results belong to the client.
Box/kader
Significantly better lead compound
The question: Optimizing an enzyme inhibitor lead compound that owes its high target affinity to two carboxylic groups
The facts: Affinity screening tests obviously showed the necessity of two carboxylic groups. By deleting one of these functional groups results affinity was lowered dramatically. Unfortunately, however, the presence of two carboxyl groups leads to poor membrane penetration and consequently to low bioavailability.
Usual approach: All initial efforts focused on chemical modification to improve membrane permeability without any concession to the carboxylic groups; they both should be preserved. Such chemical modification may include: temporary masking one of the carboxylic groups or replacing one carboxylic group by a bioisosteric group. These proposed chemical modifications typically result from a one dimensional optimisation approach: only optimising in the direction of target affinity. The result of this approach proved to be highly potent molecules with a bioavailability of less than 1%.
Our solution: We envisaged that a multidimensional optimisation approach taking into account both affinity and bioavailability would be the preferred way to go. We started by thoroughly studying the receptor, in this case the active site of the enzyme. This approach led to the insight we found an additional point of interaction in the active enzyme site. Then we replaced one of the two carboxylic groups by a functional group that exactly fits the third active site. It resulted in a significantly better compound with a somewhat lower affinity but with a bioavailability of at least 80%!
Box/kader
GPCR ligand without a basic N: alternative binding mode
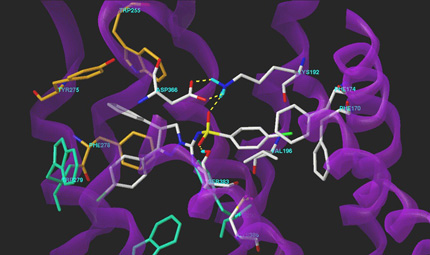
The question: Finding a highly selective GPCR lead compound
The facts: In recent decades the G-Protein Coupled Receptors (GPCRs) have extensively been investigated. For decades ligands for these targets have been a rich source of new drugs for the pharmaceutical industry. The existing paradigm was that a vital molecular request for these drugs is the presence of a basic nitrogen atom in the molecular structure.
Our solution: We performed a high throughput screening and discovered a number of weak hits that did not contain the basic nitrogen atom. Despite the existing paradigm we decided to look for opportunities to optimize these hits and discovered an alternative binding motive that resulted in equipotent molecules. These compounds showed an unprecedented selectivity for other GPCR’s and for a number of safety-related targets. The resulting optimized molecule is currently in phase II clinical studies!









