|
|

Christian Heinis |
Young researcher
Christian Heinis
Christian Heinis studied biochemistry at the Swiss Federal Institute of Technology in Zurich (ETHZ). From 2000 to 2003 he did a Ph.D. in the research group of Prof. Dr. Dario Neri at the ETH Zurich where he worked on the in vitro evolution of enzymes with therapeutic applications in mind. In 2004, he joined the group of Prof. Dr. Kai Johnsson at the Ecole Polytechnique Fédérale de Lausanne (EPFL) as a post-doctoral fellow and applied directed evolution to engineer an alkyltransferase for the use in molecular imaging. In 2006, Christian Heinis started a second post-doc in the group of Sir Greg Winter at the MRC Laboratory of Molecular Biology (LMB) in Cambridge, UK. With Sir Greg Winter, he had developed a novel method for the generation of bicyclic peptides with tailored binding specificities. In 2008, Christian Heinis was appointed tenure-track Assistant Professor at the Institute of Chemical Sciences and Engineering (ISIC) of the EPFL in Switzerland, and since 2009 he is holding a professorship of the Swiss National Science Foundation. Christian Heinis is along with Sir Greg Winter a scientific founder of the spin-off company Bicycle Therapeutics (www.bicycletherapeutics.com).
Research interest
The main research goal of Christian Heinis and his team of currently nine scientists is the development of therapeutics based on bicyclic peptides. As the name implies, bicyclic peptides are peptide structures with two macrocyclic rings. They can conveniently be obtained by connecting three amino acids in linear peptides with chemical linkers (please see the figure). The bicyclic peptides promise to combine favourable properties of two major classes of therapeutics, the monoclonal antibodies and the small molecule drugs: as antibodies, the bicyclic peptides contain conformationally constrained peptide loops that can potentially interact with extended surfaces of therapeutic targets to bind with high affinity and selectivity. As small molecules, the bicyclic peptides can be chemically synthesized, can diffuse into tissue and offer multiple application options. Christian Heinis had started to work with bicyclic peptides as a post-doctoral follow in the research group of Sir Greg Winter at the Laboratory of Molecular biology (LMB) in Cambridge, UK, where he had proposed an approach to generate phage-encoded multicyclic peptides. Sir Greg Winter and Christian Heinis had speculated that the diversity of binding sites in antibodies, which are restricted to a relatively small region (the complementary determining regions), can be mimicked by the cyclic peptide structures. Together, they had developed a methodology based on phage display that allows the generation and genetic encoding of billions of bicyclic peptides and the subsequent identification of ligands binding to targets of interest as described in the following section.
Screening of bicyclic peptide libraries by phage display
Phage display is a powerful technique widely applied for the isolation of binders from large polypeptide libraries (> a billion different polypeptides). It had led to the isolation of numerous binders based on short peptides, antibodies or diverse protein scaffolds. Some protein therapeutics developed by phage display including antibodies and a so called Kunitz domain are already in clinical use. To generate libraries of phage-encoded bicyclic peptides, linear peptides are displayed on the surface of filamentous phage and cyclised in a chemical reaction (please see the figure). For example, random peptides containing two cysteine residues at both ends and a third one in the middle are reacted with the molecule tris-(bromomethyl)benzene. In a first proof of concept experiment, the phage selection strategy was successfully applied by Sir Greg Winter and Christian Heinis to generate inhibitors of human plasma kallikrein and cathepsin G with nanomolar affinities (Heinis, C., et al., Nat. Chem. Biol., 2009). More recently, the laboratory of Christian Heinis had isolated bicyclic peptides that inhibit other proteases or bind to a receptor. One of the inhibitors was crystallized together with the protease target and its structure determined to gain insight into the binding mode of bicyclic peptides. To generate new designs of multicyclic peptides, the group of Christian Heinis is currently varying the format of the peptide component as well as applying different cyclization chemistries.
Towards the development of bicyclic peptide therapeutics
To assess the therapeutic potential of bicyclic peptides, the team of Christian Heinis is generating antagonists or agonists for various disease-related proteins and is testing their activity in biological assays and in vivo. Clinical indications considered are those in which bicyclic peptides promise advantages over monoclonal antibodies and small molecules. Possible advantages over antibodies include better tissue penetration, higher stability, manufacturing by chemical synthesis, higher activity per mass and wider choice of application routes. Advantages over small molecules can include higher binding affinity, better target specificity and ability to disrupt protein-protein interactions. Thanks to the excellent infrastructure and support of scientists of various disciplines at the EPFL, the laboratory of Christian Heinis could recently start with the in vivo testing of a bicyclic peptide, an inhibitor of a tumour-associated protease. A pharmacokinetic study gave promising results allowing the testing of this first bicyclic peptide in tumour-bearing mice. Towards the development of therapeutic bicyclic peptides, the group of Christian Heinis is also actively collaborating with the spin-off company Bicycle Therapeutics.
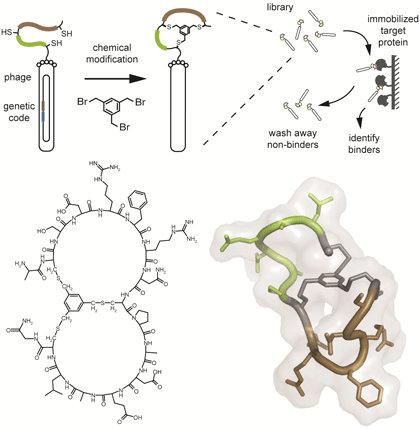
Figure
Upper panel: Schematic representation of the strategy to generate phage-encoded bicyclic peptides and to isolate binders from large combinatorial libraries. Lower panel: Structural formula and NMR structure of a bicyclic peptide. A mesitylene group connects three cysteine residues in the peptide via stable thioether bonds.
|
|

Editor
Gabriele Costantino
Univ. of Parma, IT
Editorial Committee
Erden Banoglu
Gazi Univ., TR
Lucija Peterlin Masic
Univ. of Ljubljana, SLO
Leonardo Scapozza
Univ. of Geneve, CH
Wolfgang Sippl
Univ. Halle-Wittenberg, DE
Sarah Skerratt
Pfizer, Sandwich, UK

Executive Committee
Hans-Ulrich Stilz President
Gerhard F. Ecker Past Pres.
Koen Augustyns Secretary
Rasmus P. Clausen Treasurer
Hein Coolen Member
Gabriele Costantino Member
Phil Jones Member

|









